Dyeing Preparation
Dyeing Preparation Process
What preparation is needed before dyeing cotton?
Preparation of cotton fibers, yarns, and fabrics to undergo dyeing and finishing processes is critical because many problems that appear later can be traced back to inadequate preparation.
Dyeing Preparation Steps

Mercerized Cotton Skeins
Scouring
Whatever the substrate—a fiber, a yarn, a fabric, or an entire garment—the first step of preparation is scouring to remove unwanted impurities. Scouring also improves absorbency and ensures that the cotton is uniformly clean.
Regardless of the substrate, whether it is a fiber, a yarn, fabric, or an entire garment—the first step of preparation is to scour or remove unwanted impurities. Scouring improves absorbency and ensures that the cotton is uniformly clean.
Identifying Impurities
Before you begin to scour, it is important to know what type of impurity the substrate contains and how much is present. You can learn this by:
- Laboratory testing
- Visual inspection
- Past experience
- Communication with your suppliers
Once impurities have been identified, you need to carefully consider the most efficient way to remove them based on the machine’s capabilities.
Scouring Recipe
What makes up a scouring recipe for cotton? It typically includes an alkali such as soda ash or caustic soda. This alkali swells the cotton and breaks up the moats, part of the cottonseed still in the fiber.
A scouring recipe also includes a chelating agent which ties up the metals such as iron and calcium which can come from a number of sources such as the cotton, the water, or even the machine. Included in this mix is a surfactant which is essentially a soap. A surfactant improves the penetration of the chemistry into the fiber. Surfactants help remove the impurities and prevent redepositing.
Bleaching
Bleaching produces a uniform white base, so that light or bright colors can be achieved. Various chemicals can produce the whiteness achieved by bleaching, but the most commonly used is hydrogen peroxide. Peroxide bleaching offers a distinct advantage by obtaining good whiteness and is both economical and flexible to use.
Peroxide bleaching requires other chemistry to properly execute the desired reaction. In addition to the hydrogen peroxide, a typical bleach bath contains an alkali that provides the proper pH and activates the peroxide. A stabilizer controls that activation, a sequestrant ties up metals which could accelerate the chemical reaction and weaken the fiber. A surfactant wets out the fiber and emulsifies impurities. With bleaching, it is critical that time, temperature, and pH chemistry are controlled to obtain the most efficient white and cause the least amount of damage to the fiber.
Mercerizing
Mercerization changes the physical structure, appearance, and properties of the cotton fiber. With mercerization, the substrate is exposed to a concentrated solution of caustic soda, usually under tension on a tenter frame. Mercerization changes the cotton fiber’s cross-sectional shape and morphology.
As it is mercerized, the cotton fiber changes from a kidney bean shape to a rounder shape. The caustic soda causes the cellulosic fiber to swell. As a result, the fiber becomes more open or amorphous rather than crystalline. This enables the fiber to take up more dye which makes mercerization useful when you have issues with colorfastness or you have a large amount of immature fiber.

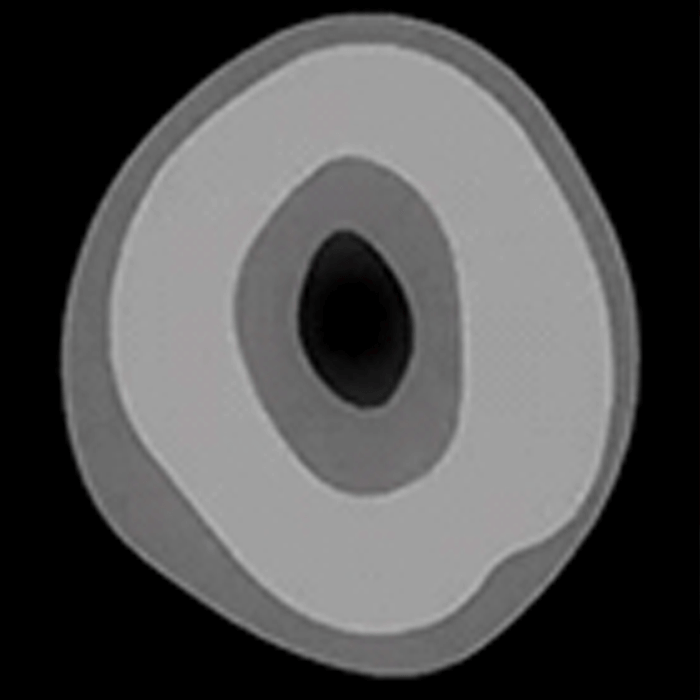
The above image shows the cross-sectional view of an unmercerized cotton fiber on the left and a mercerized cotton fiber on the right. Not only is mercerized fiber more receptive to dye, but the fabric is also stronger and displays a smoother appearance. Because the shape of the fiber has changed, the fabric reflects light more readily and if mercerized under tension, the fabric has more luster.
Rinsing & Neutralizing
The final step in the preparation of any substrate is rinsing and neutralizing any chemistry that has been used. To ensure that the fiber is clean, the solubilized matter created during scouring must be removed. The final pH of the substrate must be neutral. You accomplish this by removing any residual alkalinity that remains after either scouring, mercerizing, or bleaching. If the material has been bleached, peroxide must be removed. A properly prepared substrate can help ensure that the dyes or finishes to be applied later are uniform. Processes or procedures may vary but how they occur must be carefully controlled in order to perform a consistent preparation step. You can regularly conduct standardized tests to measure pH, total alkalinity, absorbency, barium number, and the whiteness index. Document all processes carefully and investigate and isolate any variations in the results so corrections may be made.


